The ICMJE’s clinical trial registration policy is detailed in a series of editorials (see News and Editorials and FAQs).
Briefly, the ICMJE requires, and recommends that all medical journal editors require, registration of clinical trials in a public trials registry at or before the time of first patient enrollment as a condition of consideration for publication. Editors requesting inclusion of their journal on the ICMJE website list of publications that follow ICMJE guidance should recognize that the listing implies enforcement by the journal of ICMJE’s trial registration policy.
ICMJE uses the date trial registration materials were first submitted to a registry as the date of registration. When there is a substantial delay between the submission of registration materials and their posting at the trial registry, editors may inquire about the circumstances that led to the delay.
The ICMJE defines a clinical trial as any research project that prospectively assigns people or a group of people to an intervention, with or without concurrent comparison or control groups, to study the relationship between a health-related intervention and a health outcome. Health-related interventions are those used to modify a biomedical or health-related outcome; examples include drugs, surgical procedures, devices, behavioural treatments, educational programs, dietary interventions, quality improvement interventions, and process-of-care changes. Health outcomes are any biomedical or health-related measures obtained in patients or participants, including pharmacokinetic measures and adverse events. The ICMJE does not define the timing of first participant enrollment, but best practice dictates registration by the time of first participant consent.
The ICMJE accepts publicly accessible registration in any registry that is a primary register of the WHO International Clinical Trials Registry Platform (ICTRP) that includes the minimum acceptable 24-item trial registration data set or in ClinicalTrials.gov, which is a data provider to the WHO ICTRP. The ICMJE endorses these registries because they meet several criteria. They are accessible to the public at no charge, open to all prospective registrants, managed by a not-for-profit organization, have a mechanism to ensure the validity of the registration data, and are electronically searchable. An acceptable registry must include the minimum 24-item trial registration data set (http://prsinfo.clinicaltrials.gov/trainTrainer/WHO-ICMJE-ClinTrialsgov-Cross-Ref.pdf or www.who.int/clinical-trials-registry-platform) at the time of registration and before enrollment of the first participant. The ICMJE considers inadequate trial registrations missing any of the 24 data fields, those that have fields that contain uninformative information, or registrations that are not made publicly accessible such as phase I trials submitted to the CTIS (Clinical Trials Information System) and trials of devices for which the information is placed in a “lock box.” In order to comply with ICMJE policy, investigators registering trials of devices at ClinicalTrials.gov must “opt out” of the lock box by electing public posting prior to device approval. Approval to conduct a study from an independent local, regional, or national review body (e.g., ethics committee, institutional review board) does not fulfill the ICMJE requirement for prospective clinical trial registration. Although not a required item, the ICMJE encourages authors to include a statement that indicates that the results have not yet been published in a peer-reviewed journal, and to update the registration with the full journal citation when the results are published.
The purpose of clinical trial registration is to prevent selective publication and selective reporting of research outcomes, to prevent unnecessary duplication of research effort, to help patients and the public know what trials are planned or ongoing into which they might want to enroll, and to help give ethics review boards considering approval of new studies a view of similar work and data relevant to the research they are considering. Retrospective registration, for example at the time of manuscript submission, meets none of these purposes. Those purposes apply also to research with alternative designs, for example observational studies. For that reason, the ICMJE encourages registration of research with non-trial designs, but because the exposure or intervention in non-trial research is not dictated by the researchers, the ICMJE does not require it.
Secondary data analyses of primary (parent) clinical trials should not be registered as separate clinical trials, but instead should reference the trial registration number of the primary trial.
The ICMJE expects authors to ensure that they have met the requirements of their funding and regulatory agencies regarding aggregate clinical trial results reporting in clinical trial registries. It is the authors’, and not the journal editors’, responsibility to explain any discrepancies between results reported in registries and journal publications. The ICMJE will not consider as prior publication the posting of trial results in any registry that meets the above criteria if results are limited to a brief structured abstract or tables (to include trial participants enrolled, baseline characteristics, primary and secondary outcomes, and adverse events).
The ICMJE recommends that journals publish the trial registration number at the end of the abstract. The ICMJE also recommends that, whenever a registration number is available, authors list this number the first time they use a trial acronym to refer either to the trial they are reporting or to other trials that they mention in the manuscript.
Editors may consider whether the circumstances involved in a failure to appropriately register a clinical trial were likely to have been intended to or resulted in biased reporting. Because of the importance of prospective trial registration, if an exception to this policy is made, trials must be registered and the authors should indicate in the publication when registration was completed and why it was delayed. Editors should publish a statement indicating why an exception was allowed. The ICMJE emphasizes that such exceptions should be rare, and that authors failing to prospectively register a trial risk its inadmissibility to our journals.
The ICMJE’s data sharing statement policy is detailed in an editorial (see Updates and Editorials).
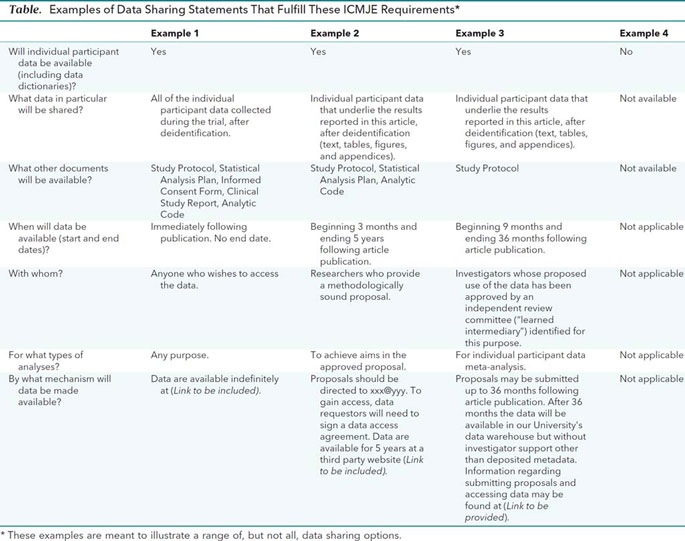
Data sharing statements must indicate the following: whether individual deidentified participant data (including data dictionaries) will be shared (“undecided” is not an acceptable answer); what data in particular will be shared; whether additional, related documents will be available (e.g., study protocol, statistical analysis plan, etc.); when the data will become available and for how long; by what access criteria data will be shared (including with whom, for what types of analyses, and by what mechanism). Illustrative examples of data sharing statements that would meet these requirements are provided in the Table.

Authors of secondary analyses using shared data must attest that their use was in accordance with the terms (if any) agreed to upon their receipt. They must also reference the source of the data using its unique, persistent identifier to provide appropriate credit to those who generated it and allow searching for the studies it has supported. Authors of secondary analyses must explain completely how theirs differ from previous analyses. In addition, those who generate and then share clinical trial data sets deserve substantial credit for their efforts. Those using data collected by others should seek collaboration with those who collected the data. As collaboration will not always be possible, practical, or desired, the efforts of those who generated the data must be recognized.